Aromatic Nucleophilic Substitution
Aromatic nucleophilic substitution is an extremely useful tool for the functionalisation of aryl compounds. It has been around since the early 1950’s and is still very common in organic synthesis practice, although more modern methods to couple nitrogen and aromatic carbon, such as the Buchwald-Hartwig cross-coupling, have been gaining popularity in the last decade.
As with all substitution reactions, a nucleophile such as an amine or an alcohol displaces a leaving group on the aromatic ring, this usually being a halide.
There are several different mechanisms associated to the aromatic nucleophilic substitution. Only three have practical relevance to organic synthesis:
- Addition-elimination mechanism
- Benzyne mechanism
- Aromatic SN1 of diazonium salts
The SNAr via addition-elimination mechanism is the most common reaction, occurring in the case of most substituted aryl halides. The electron withdrawing effect of the substituent facilitates the addition of the nucleophile on the ring by stabilising the negatively charged intermediates before the elimination of the leaving group.

The Benzyne mechanism is relevant to unsubstituted aryl halides and proceeds via a benzyne intermediate as per the below scheme.

Finally the Aromatic SN1 occurs in the case of aromatic diazonium salts via the formation of an aryl cation intermediate by elimination of N2 and the subsequent addition of the nucleophile to give the substitution product.
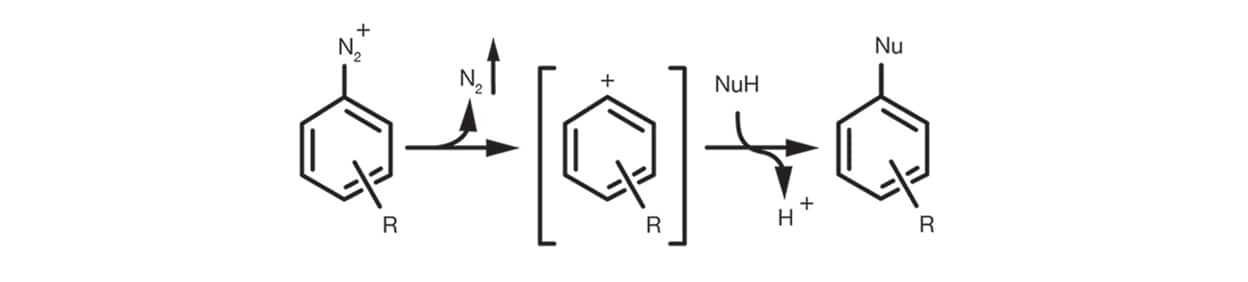
The reaction setup is quite simple. It is usually carried out in relatively polar organic solvents, protic and aprotic, such as DMSO, ethanol, DMF, NMP. It is supported by the use of an auxiliary base (tertiary amines and carbonates are the most common) and rarely needs significant excesses of nucleophile.
Reference Reaction Protocols
1.
Weigh equimolar amounts of aryl halide and amine (5-50mmol). Add 1.5-2 equivalents of potassium carbonate (or alternative base) in ethanol. Heat to reflux for 8-24h.
Aniline derivatives can usually be easily precipitated by pH shift.
2.
Weigh equimolar amounts of aryl halide and alcohol, dissolve in THF and suspend 1.5 equivalents of sodium hydride. Stir for 8-24h. The reaction can proceed at room temperature, however it is often necessary to heat up to 80-100°C.
Ether products are usually separated by extraction with water, polar or apolar solvents. Magnesium sulfate is commonly used to remove water and vacuum to remove volatiles.
Thiols can be effectively used as nucleophiles and the reaction protocol is typically the same as for the alcohols.
3.
Dissolve the aryl halide, a slight excess of potassium cyanide (1.1-1.3 equivalents) and an equimolar amount of trimethylamine in DMSO. Stir the reaction at about 50°C for 12-24h adding water dropwise.
Reaction products can be diluted in water and extracted with ethyl acetate, or other aprotic polar solvents.
Chromatographic purification is often necessary.
Examples
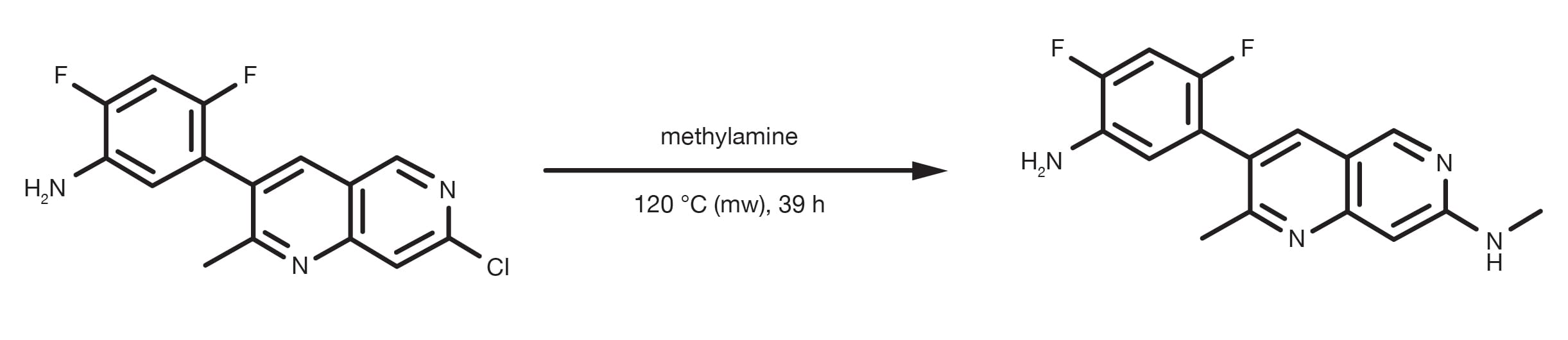
Patent Reference: WO2013134298

Patent Reference: WO2014149164

Patent Reference: WO2016012477
Key literature references
- Chem. Rev., 1951, 49 (2): 273–412. https://doi.org/10.1021/cr60153a002
- Molecules 2006, 11, 130-133. https://doi.org/10.3390/11020130
Product Selection
Solvents:
Basic ingredients / Additives:
Bases:
Potassium tert-butoxide solution
1,4-diazabicyclo[2.2.2]octane (DABCO)
Catalysts:
Silica gelcan be used as catalyst in some high temperature reactions of unsubstituted chlorobenzene
Building blocks:
(poly)substituted aryl halides:
N-Heteroaryl halides:
4-Chloropyridine hydrochloride
(hetero)aryl diazonium salts:
4-Nitrobenzenediazonium tetrafluoroborate
4-Methoxybenzenediazonium tetrafluoroborate
4-Bromobenzenediazonium tetrafluoroborate
Nucleophiles:
amines (primary, secondary and tertiary)
Aromatic alcohols (variously substituted phenols):
Thiols:
Other nucleophiles:
